Answer:
15.2 mL
Step-by-step explanation:
First we calculate the volume of the copper
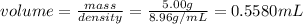
when this volume of copper is added to the water, the water level will rise by the level of the volume of copper. So the final volume is:
14.6 mL + 0.5580 mL = 15.158 mL
Since the measuring cylinder is graduated to one decimal place , we can round this up to 15.2 mL