The rate law for the overall reaction is:
![\[ \text{Rate}_{\text{overall}} = k_{\text{overall}}[A]^2[B] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/16r4bcqjz2aehn06vzl4rmc5lm62lwpl3v.png)
How to determine rate law?
The given reaction mechanism involves two steps:
![\[ \text{Step 1: } A + B \underset{k_1}{\overset{k_(-1)}{\rightleftharpoons}} C \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/q8s35op4820g9tywhlx3ncv3kjf3uerovx.png)
![\[ \text{Step 2: } C + A \xrightarrow{k_2} D \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/6elyxx1tesunhsem21084f0eykawyk0w9l.png)
The overall reaction is the sum of these steps:
![\[ 2A + B \xrightarrow{k_{\text{overall}}} D \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/urpgmc73ikkux4wd6lbnb0f8hegjhrcpq4.png)
To determine the rate law for the overall reaction, consider the rate-determining step, which is the slowest step in the mechanism. In this case, it's Step 2.
The rate law for Step 2 is given by:
![\[ \text{Rate}_{\text{Step 2}} = k_2[C][A] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/i7xn810p0f4v4wi81at395jf6qg2qp7pf6.png)
At equilibrium for Step 1:
![\[ K_{\text{eq}} = ([C])/([A][B]) \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/l90oufox0py7mbagmb5t9mvcjadbb1z3a2.png)
Now, rearrange the equation to solve for [C]:
![\[ [C] = K_{\text{eq}}[A][B] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ihj0yu8dlnw1otlx44yyilfz14f604hga6.png)
Now substitute this expression for [C] into the rate law for Step 2:
![\[ \text{Rate}_{\text{Step 2}} = k_2K_{\text{eq}}[A][B][A] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/cz8o4lwixz0mead3sgyu9i7guyt3kc9pwi.png)
Combine like terms:
![\[ \text{Rate}_{\text{Step 2}} = k_{\text{overall}}[A]^2[B] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/l6hyhvfomrm8h8dh5kha4n61sdv20jf2bw.png)
Therefore, the rate law for the overall reaction is:
![\[ \text{Rate}_{\text{overall}} = k_{\text{overall}}[A]^2[B] \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/16r4bcqjz2aehn06vzl4rmc5lm62lwpl3v.png)
The overall rate constant
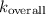 incorporates the rate constants for both steps.
incorporates the rate constants for both steps.