Answer:
73.5 kJ
Explanation:
Mass of butane = 185 g
Heat of vaporization for butane = 23.1 kJ/mol
Molar mass of butane = 58.12 g/mol
Number of moles of butane =
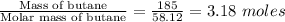
Energy required for burning 185 g of butane = 3.18×23.1 = 73.5 kJ
∴ Energy is required to vaporize 185 g of butane at its boiling point is 73.5 kJ