Answer:
0.558mole of SO₃
Step-by-step explanation:
Given parameters:
Molar mass of SO₃ = 80.0632g/mol
Mass of S = 17.9g
Molar mass of S = 32.065g/mol
Number of moles of O₂ = 0.157mole
Molar mass of O₂ = 31.9988g/mol
Unknown:
Maximum amount of SO₃
Solution
We need to write the proper reaction equation.
2S + 3O₂ → 2SO₃
We should bear in mind that the extent of this reaction relies on the reactant that is in short supply i.e limiting reagent. Here the limiting reagent is the Sulfur, S. The oxygen gas would be in excess since it is readily availbale.
So we simply compare the molar relationship between sulfur and product formed to solve the problem:
First, find the number of moles of Sulfur, S:
Number of moles of S =
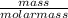
Number of moles of S =
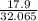 = 0.558mole
= 0.558mole
Now to find the maximum amount of SO₃ formed, compare the moles of reactant to the product:
2 mole of Sulfur produced 2 mole of SO₃
Therefore; 0.558mole of sulfur will produce 0.558mole of SO₃