Answer: The unknown gas is B)
 .
.
Explanation: The problem is based on Graham's law of effusion rates of gases.
Carbon dioxide takes 4.69 times as long to escape as the unknown gas. It means the unknown gas is lighter than carbon dioxide since the lighter gas takes less time to escape.
From Graham's law, "Rate of effusion of a gas is inversely proportional to the molar mass of the gas."
Molar mass of carbon dioxide is 44.0 gram per mol. Let's say the molar mass of the unknown gas is M.
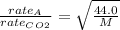
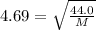
Do the square to both sides:
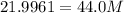
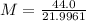
M = 2.00
2.00 gram per mol is the molar mass of hydrogen gas. So, the correct option is B)
 .
.