Answer:
The inventor's claim is false in the sense that no thermal machine can violate the first thermodynamic law.
Step-by-step explanation:
The inventor's claim could not be possible as no thermal machine can transfer more heat than the input work consumed. If we expose the thermal efficiency:
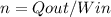
Where Q and W both must be in the same power unit, so we will convert the remove heat from BTU/hr to hp:
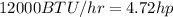
Therefore by comparing, we notice that the removing heat of 4.75 hp is large than the delivered work of 1.11 hp. By evaluating the efficiency:
[tex]n=4.75 hp / 1.1 hp = 4.3 > 1[/tex]