Answer: Option (B) is the correct answer.
Step-by-step explanation:
A chemical reaction in which products can be changed back into the reactants is known as a reversible reaction.
For example,
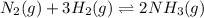
On the other hand, a chemical reaction in which products are not changed back into the reactants then it is known as an irreversible reaction.
For example,
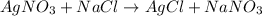
Thus, we can conclude that there are some reactions, however, that can return to their original forms. These reactions are called reversible.