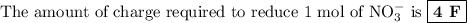Answer:
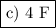
Step-by-step explanation:
1. Write the skeleton equation for the half-reaction
NO₃⁻ ⟶ N₂O
2. Balance all atoms other than H and O
2NO₃⁻ ⟶ N₂O
3. Balance O by adding H₂O molecules to the deficient side.
2NO₃⁻ ⟶ N₂O + 5H₂O
4. Balance H by adding H⁺ ions to the deficient side.
2NO₃⁻ + 10H⁺ ⟶ N₂O + 5H₂O
5. Balance charge by adding electrons to the deficient side.
2NO₃⁻ + 10H⁺ + 8e⁻ ⟶ N₂O + 5H₂O
The amount of charge required to reduce 2 mol of NO₃⁻ is 8 F