Answer: The amount of I-125 after the given time is 5.002 mg.
Step-by-step explanation:
All the radioactive reactions follows first order kinetics.
The equation used to calculate half life for first order kinetics:
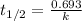
We are given:
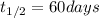
Putting values in above equation, we get:
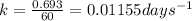
Rate law expression for first order kinetics is given by the equation:
![k=(2.303)/(t)\log([A_o])/([A])](https://img.qammunity.org/2020/formulas/chemistry/college/67w3lufh8bppkbbcp1xaut7f9029vt6k0l.png)
where,
k = rate constant =
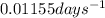
t = time taken for decay process = 240 days
![[A_o]](https://img.qammunity.org/2020/formulas/chemistry/high-school/38eb24kf04xqy5t88y9g0vzh3m04r4nqgg.png) = initial amount of the reactant = 80 mg
= initial amount of the reactant = 80 mg
[A] = amount left after decay process = ?
Putting values in above equation, we get:
![0.01155days^(-1)=(2.303)/(240days)\log(80)/([A])](https://img.qammunity.org/2020/formulas/chemistry/college/xrytixatrqem0db3txt74fctv8n8p6dpax.png)
![[A]=5.002mg](https://img.qammunity.org/2020/formulas/chemistry/college/4leffuo9ppoj90asok7dfclnksimew4ksg.png)
Hence, the amount of I-125 after the given time is 5.002 mg.