Answer:
The wood from boat is 17190 years old
Step-by-step explanation:
For first order radioactive decay-
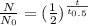
where N is remaining amount of radioactive elements after "t" time,
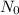 is initial amount of radioactive element and
is initial amount of radioactive element and
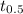 is half-life of radioactive element
is half-life of radioactive element
Here
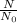 is 0.125,
is 0.125,
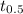 is 5730 years
is 5730 years
Plug-in all the values in the above equation-
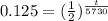
or, t = 17190
so t is 17190 years or wood from boat is 17190 years old