Answer : The oxidation state of manganese (Mn) is, (+7)
Explanation :
Oxidation number or oxidation state : It represent the number of electrons lost or gained by the atoms of an element in a compound.
Oxidation numbers are generally written with the sign (+) and (-) first and then the magnitude.
When the atoms are present in their elemental state then the oxidation number will be zero.
The oxidation number of oxygen (O) in compounds is usually -2.
The given compound is,
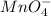
Let the oxidation state of 'Mn' be, 'x'
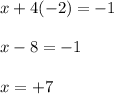
Therefore, the oxidation number of manganese (Mn) is, (+7)