Answer: The correct answer is Option a.
Step-by-step explanation:
The reaction between sodium thiosulfate and nitric acid produces a number of products. The balanced chemical equation for the reaction of two follows:
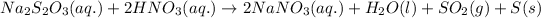
By Stoichiometry of the reaction:
1 mole of sodium thiosulfate reacts with 2 moles of nitric acid to produce 2 moles of sodium nitrate, 1 mole of water, 1 moles of sulfur dioxide and 1 mole of solid sulfur.
Hence, the correct answer is Option a.