Answer: The percentage purity of sample is 87.54 %.
Step-by-step explanation:
Net ionic equation of any reaction does not include any spectator ions.
Spectator ions are defined as the ions which does not get involved in a chemical equation. They are found on both the sides of the chemical reaction when it is present in ionic form.
The chemical equation for the reaction of ammonium nitrate and sodium hydroxide is given as:
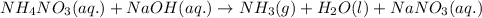
Ionic form of the above equation follows:
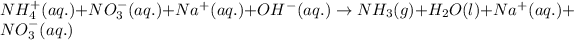
As, sodium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
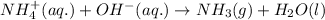
To calculate the moles of sodium hydroxide, we use the equation:
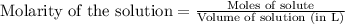
Molarity of sodium hydroxide = 0.1023 M
Volume of sodium hydroxide = 22.62 mL = 0.02262 L (Conversion factor: 1 L = 1000 mL)
Putting values in above equation, we get:
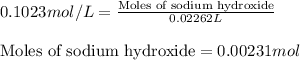
For the given chemical equation:
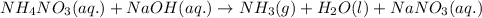
By Stoichiometry of the reaction:
1 mole of sodium hydroxide reacts with 1 mole of ammonium nitrate.
So, 0.00231 moles of sodium hydroxide will react with =
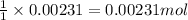 of ammonium nitrate.
of ammonium nitrate.
- To calculate the mass of ammonium nitrate, we use the equation:

Moles of ammonium nitrate = 0.00231 mol
Molar mass of ammonium nitrate = 80 g/mol
Putting values in above equation, we get:
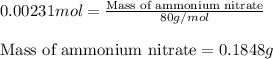
- To calculate the percentage purity of ammonium nitrate, we use the equation:
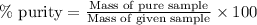
Mass of pure sample = 0.1848 g
Mass of given sample = 0.2111 g
Putting values in above equation, we get:
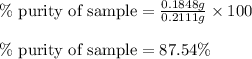
Hence, the percentage purity of sample is 87.54 %.