Answer:
d. The overall free-energy change must be negative
Step-by-step explanation:
For a reaction to be spontaneous, The overall free-energy change must be negative for the reaction.
ΔG of a reaction can be calculated by subtracting the free energy of reactants from the free energy of products.
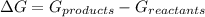
Therefore, if a chemical reaction undergoes from a higher value of free energy (Reactants) to a lower value of free energy (Products), then ΔG will be negative in magnitude and the chemical reaction will be spontaneous.
Thus, ATP involved, Phosphate group transferred or a common intermediate must be formed or not, if free energy change is negative, the reaction will be spontaneous.