Answer:
m =8.81*10^{-6}grams
time t = 52.8 year
Step-by-step explanation:
GIVEN DATA:
the half life of the CO-60 is, T_1/2 = 5.27 years = 1.663 e+8 s
activity dN/dt = 1 mCi = 3.7 X 10^7 decay/s
activity ,
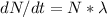
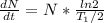
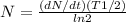
= ( 3.7 X 10^7 )(1.663*10^8 ) / ln2
= 8.877*10^{16}
Number of moles:
n = N/NA = 8.877*10^{16} / 6.022X10^23 = 1.474*10^{-7} mol
mass of the CO-60 is,
m = n*M = [1.474*10^{-7} mol]*[59.93 grams /mol] = 8.81*10^{-6}grams
-----------------------------------------------------------------------------------------
time t = -[T1/2 / ln2]*ln[N/N0]
= - [5.3 years / ln2]*ln[1x10-6/1x10-3]
= 52.8 year