Answer:
0.167 J/(g °C)
Step-by-step explanation:
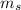 = mass of the sample = 250 g
= mass of the sample = 250 g
 = Initial temperature of sample = 80 °C
= Initial temperature of sample = 80 °C
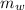 = mass of the water = 300 g
= mass of the water = 300 g
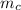 = mass of the cup = 100 g
= mass of the cup = 100 g
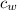 = Specific heat of water = 4.186 J/(g °C)
= Specific heat of water = 4.186 J/(g °C)
 = Specific heat of sample = ?
= Specific heat of sample = ?
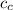 = Specific heat of aluminum cup = 0.9 J/(g °C)
= Specific heat of aluminum cup = 0.9 J/(g °C)
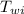 = Initial temperature of water = 20 °C
= Initial temperature of water = 20 °C
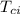 = Initial temperature of aluminum cup = 20 °C
= Initial temperature of aluminum cup = 20 °C
 = Final temperature = 21.8 °C
= Final temperature = 21.8 °C
Using conservation of heat
Heat lost by sample = Heat gained by water + Heat gained by cup


 = 0.167 J/(g °C)
= 0.167 J/(g °C)