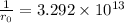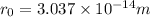Answer:
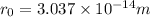
Step-by-step explanation:
Given
charge on alpha particle=+2e
mass of alpha particle=
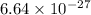 kg
kg
Charge on gold nucleus=+79e
Velocity at r=1m is

Using Energy conservation
Kinetic energy of particle will be converting to Potential energy as it approaches to nucleus
therefore
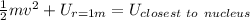
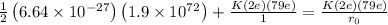
![(1)/(2)\left ( 6.64* 10^(-27)\right )\left ( 1.9* 10^(7)^2\right )=(9* 10^9* 158* \left ( 1.6* 10^(-19)\right ))/(y)\left [(1)/(r_0)-(1)/(1)\right ]](https://img.qammunity.org/2020/formulas/physics/college/9u9ci4pw2mkgkpjxu2bquacnviwcmuc57h.png)
on solving we get