Answer:
humidity = 4 × 10⁻³ kg/m³
Step-by-step explanation:
mass of water = 10.0 Kg
volume of air contained in space station = 2500 m³
density of water = 1000 kg/m³
volume of water = mass / density
=
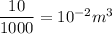
total volume = volume of air +volume of water
= 10⁻² + 2500 = 2500.01 m³
humidity (H)=
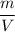
=
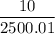
= 4 × 10⁻³ kg/m³