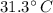Answer:
The final temperature is
Step-by-step explanation:
Specific heat capacity of aluminum ,
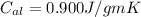
Specific heat capacity of water,
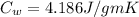
Specific heat capacity of copper ,
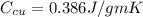
Let the final temperature be T degree C
Since Heat lost by aluminium = Heat gained by water and calorimeter
Therefore
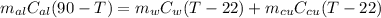
=>
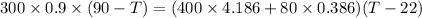
=>
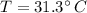
Thus the final temperature is