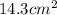Answer:
The area of the gold foil is
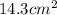
Step-by-step explanation:
Mass of gold foil , m= 27.63 g
Thickness of gold foil ,
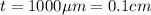
Density of gold ,
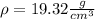
Let the area of gold foil be A then the total volume of gold foil is given by
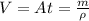
=>
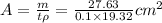
=>
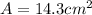
Thus the area of the gold foil is