Answer:
5.76 J/K
Step-by-step explanation:
Mole fraction of Ne at 500 K= 50 % = 0.5 =
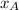
Mole fraction of Ar at 500 K = 50 %= 0.5 =
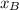
R = Gas constant = 8.314 J/Kmol
As mass is not given the number of moles of Ne and Ar are taken as 0.5
Entropy of mixture
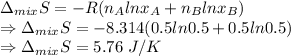
∴ Entropy of mixture is 5.76 J/K