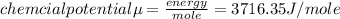Answer:
internal energy E 3716.35 j
cv = 12.47 J/K
S = 12.47 J/K
A = 0.29 J
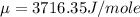
Step-by-step explanation:
given data:
Kr atomic number = 36
degree of freedom = 3
1) internal energy E =
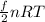
=
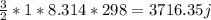
2)
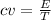
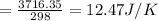
3)
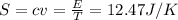
4) A, Halmholtz free energy = E -TS = 37146.35 - 12.47*298 = 0.29 J
5)