Answer:
![\mu _j=(1)/(C_p)\left [T\left((\partial v)/(\partial T)\right)_p-v\right]dp](https://img.qammunity.org/2020/formulas/physics/college/yy39839jlylb20etvptnj2as6hedk493w5.png)
Step-by-step explanation:
Joule -Thompson effect
Throttling phenomenon is called Joule -Thompson effect.We know that throttling is a process in which pressure energy will convert in to thermal energy.
Generally in throttling exit pressure is low as compare to inlet pressure but exit temperature maybe more or less or maybe remains constant depending upon flow or fluid flow through passes.
Now lets take Steady flow process
Let
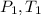 Pressure and temperature at inlet and
Pressure and temperature at inlet and
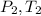 Pressure and temperature at exit
Pressure and temperature at exit
We know that Joule -Thompson coefficient given as
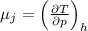
Now from T-ds equation
dh=Tds=vdp
So
![Tds=C_pdt-\left [T\left((\partial v)/(\partial T)\right)_p\right]dp](https://img.qammunity.org/2020/formulas/physics/college/g3f7woxt54jgqa5zna7pzxahds2ayuy2n8.png)
⇒
![dh=C_pdt-\left [T\left((\partial v)/(\partial T)\right)_p-v\right]dp](https://img.qammunity.org/2020/formulas/physics/college/2lhqidgwaw7w0hjt3j9o1abfwbly7ezcub.png)
So Joule -Thompson coefficient
![\mu _j=(1)/(C_p)\left [T\left((\partial v)/(\partial T)\right)_p-v\right]dp](https://img.qammunity.org/2020/formulas/physics/college/yy39839jlylb20etvptnj2as6hedk493w5.png)
This is Joule -Thompson coefficient for all gas (real or ideal gas)
We know that for Ideal gas Pv=mRT
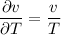
So by putting the values in
![\mu _j=(1)/(C_p)\left [T\left((\partial v)/(\partial T)\right)_p-v\right]dp](https://img.qammunity.org/2020/formulas/physics/college/yy39839jlylb20etvptnj2as6hedk493w5.png)
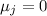 For ideal gas.
For ideal gas.