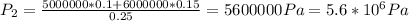Answer:
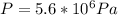
Step-by-step explanation:
Consider that, as the system is adiabatic,
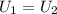 where U1 and U2 are the internal energies before the process and after that respectively.
where U1 and U2 are the internal energies before the process and after that respectively.
Consider that:
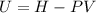 , and that the internal energy of the first state is the sum of the internal energy of each tank.
, and that the internal energy of the first state is the sum of the internal energy of each tank.
So,
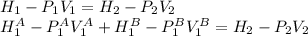
Where A y B are the tanks. The enthalpy for an ideal gas is only function of the temperature, as the internal energy is too; so it is possible to assume:
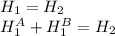
So,
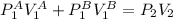
Isolating
 ,
,
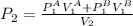
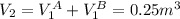
So,