Given:
mass of water, m = 2000 kg
temperature, T =
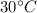 = 303 K
= 303 K
extacted mass of water = 100 kg
Atmospheric pressure, P = 101.325 kPa
Solution:
a) Using Ideal gas equation:
PV = m
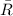 T (1)
T (1)
where,
V = volume
m = mass of water
P = atmospheric pressure
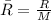
R= Rydberg's constant = 8.314 KJ/K
M = molar mass of water = 18 g/ mol
Now, using eqn (1):

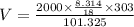
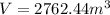
Therefore, the volume of the tank is
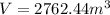
b) After extracting 100 kg of water, amount of water left, m' = m - 100
m' = 2000 - 100 = 1900 kg
The remaining water reaches thermal equilibrium with surrounding temperature at T' =
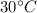 = 303 K
= 303 K
At equilibrium, volume remain same
So,
P'V = m'
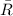 T'
T'
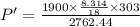
Therefore, the final pressure is P' = 96.258 kPa