Answer:
299.36 kPa
Step-by-step explanation:
given mass of steam =2 kg
initial pressure that is
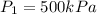
initial temperature that is
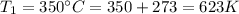
final temperature that is
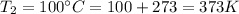
it is a rigid tank so volume is constant
for constant volume process
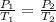
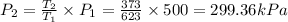
so the final pressure will be 299.36 kPa