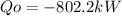Answer:
The rate at the heat is rejected from the power plant is -802.2 kW
Step-by-step explanation:
From the first thermodynamics law:
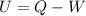
Where ΔU is the change in internal energy, ΔQ is the difference between the inlet heat and the outlet heat and ΔW is the difference between the work doing by the system and the work doing over the system:
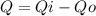
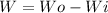
Considering a system in equilibrium the variation of energy is null:
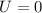
Note that it is not doing work over the system, therefore, Wi is also null.
So, for our purpose we must find the heat rejected, thas is Qo. Take into account that as the power plant is been cooled then the inlet heat is negative:
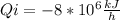
By converting the heat to Watt unit:
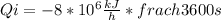
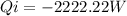
The work calculated in watts is:
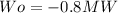
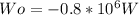
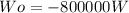
Solving first thermodynamics law for Qo, and replacing the work and the heat calculated:
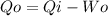
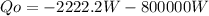
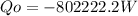
In kW the heat is: