Answer:
-1078 kJ/mol.
Step-by-step explanation:
Consider this reaction in three steps:
- Break everything down into atoms: 2 Na(g) + 2 H(g) + 2 Cl(g);
- Turn 2 Na(g) and 2 Cl(g) into 2 Na⁺(g) and 2 Cl⁻(g);
- Combine everything to form 2 NaCl(s) and H₂(g).
Sources of enthalpy changes in the first step:
- Sublimate 2 Na(s);
- Break two H-Cl bonds.
That corresponds to an enthalpy change of
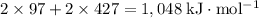 .
.
Sources of enthalpy changes in the second step:
- Remove one electron for each of the two Na(g);
- Add one electron to each of the two Cl(g).
That corresponds to an enthalpy change of
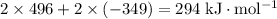 .
.
Sources of enthalpy changes in the third step:
- Bring 2 Na⁺(g) and 2 Cl⁻(g) together to form 2 NaCl(s) (which corresponds to twice the lattice enthalpy of NaCl);
- Bring 2 H(g) together to form one H₂(g).
That corresponds to an enthalpy change of
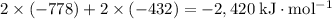 .
.
Take the sum of the enthalpy changes of the three steps to find the enthalpy change of the overall reaction:
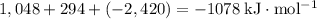 .
.