Answer:
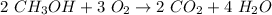
The coefficients would be 2, 3, 2, and 4.
Step-by-step explanation:
If we want to check this we would multiply the coefficient by each element. What we want is to have the same number of atoms on each side.
Reactant side:
- # of carbon atoms: 2
- # of hydrogen atoms: 8
- # of oxygen atoms: 8
Product side:
- # of carbon atoms: 2
- # of hydrogen atoms: 8
- # of oxygen atoms: 8
And that's it!