Answer:
V₂ = 225 m^{3}
so no exact option is match with the calculated answer.
Step-by-step explanation:
According to Boyle's Law, we have
P_1 V_1 = P_2 V_2 ----------- (1)
Data Given;
P_1 = 1.0 atm
V_1 = 180 m³
P_2 = 0.80 atm
V_2 = ?
Solving equation 1 for V₂,
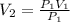
Putting values,
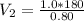
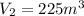
so no exact option is match with the calculated answer.