Answer:
84.4 %
Step-by-step explanation:
Let the activity of carbon is dN / dt.
Us ethe law of radioactivity
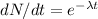
Where, λ is decay constant and t be the time
λ = 0.6931 / T
Where, T be the halflife
λ = 0.6931 / 5730 per year
λt = 0.6931 x 1400 / 5730 = 0.1693
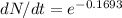
dN/dt = 0.844
it becomes 84.4%.