Answer:
Depression in freezing point = 2 X 1.853 X 0.25 = 0.9625
Thus this will be the difference between the freezing point of pure water and the solution.
Step-by-step explanation:
On adding any non volatile solute to a solvent its boiling point increases and its freezing point decreases [these are two of the four colligative properties].
The depression in freezing point is related to molality of solution as:
ΔTf
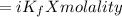
where
ΔTf= depression in freezing point
Kf= cryoscopic constant of water = 1.853 K. kg/mol.
i = Van't Hoff factor = 2 ( for KCl)
molality =
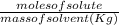
moles of solute = mass / molarmass = 4.66 / 74.55 =0.0625
mass of solvent = mass of solution (almost)
considering the density of solution to be 1g/mL
mass of solvent = 250 grams = 0.250 Kg
molality =
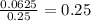
Putting values
depression in freezing point = 2 X 1.853 X 0.25 = 0.9625
Thus this will be the difference between the freezing point of pure water and the solution.