Answer:
mass of calcium chloride = 290.49g
mass of water = 494.61 g
Step-by-step explanation:
The percentage of calcium chloride in the mixture is 37.0%
It means in each 100g of mixture the mass of calcium chloride = 37.0 g
if the total mass of the mixture = 785.1 g
the mass of calcium chloride =
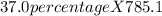
=
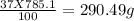
The mass of water = 785.1-290.49 = 494.61 grams