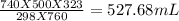Answer:
527.68 mL
Step-by-step explanation:
We will assume that nitrogen is behaving as ideal gas here.
For ideal gas the gas law is:
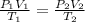
Where
P1= initial pressure = 740 torr
V1= initial volume = 500mL
T1= initial temperature = 25⁰C = 298 K
P2= final pressure = 760 torr
V2= final volume = ?
T2= final temperature = 50⁰C = 323 K
Putting values in the gas law
Final volume =