Answer:
0.187kg
Step-by-step explanation:
Given parameters:
Percentage compostion of bauxite in ore = 54%
Mass of ore = 1kg
Unknown:
Mass of Al that can be extracted from the ore = ?
Solution
Bauxite is an ore of Aluminium. It is made up of several minerals from with aluminium can be extracted in economic amount. Some of these minerals are gibbsite, boehmite, diaspore etc. Let us assume that the ore we are dealing with contains chiefly gibbsite.
The formula of Gibbsite is Al(OH)₃
We know that 54% of the ore is made up of bauxite and the remaining 46% of the ore will form the gangue(not useful part of the ore)>
Let us find the mass of the bauxite contained in the ore:
Mass of bauxite =
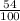 x 1kg = 0.54kg
x 1kg = 0.54kg
Since we know the mass of bauxite in the ore now, we can estimate the mass of the aluminium in the ore.
First, we find the molar mass of the gibbsite:
Molar mass of Al(OH)₃ = 27 + 3(16 + 1) = 78gmol⁻¹
Atomic mass of aluminium is 27
Mass of aluminium that could be extracted =
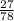 x 0.54
x 0.54
= 0.346 x 0.54
= 0.187kg