Answer:
Step-by-step explanation:
Given parameters:
Mass of Na = 1kg = 1000g
Mass of Br = 3kg = 3000g
Unkown:
Limiting reagent =?
Amount of excess reagent = ?
Quantity of NaBr formed = ?
Solution
The reaction equation can be expressed as:
2Na + Br₂ → 2NaBr
To find the limiting reagent
The limiting reagent is the reagent that is in short supply and it determines the amount of products that can be formed in the reaction.
To find the limiting the reagent, we establish a mole relationship between the reactants. We then check their stoichoimetric expression to see the one in excess and the limiting reagent.
Number of moles of Na =
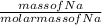
molar mass is expressed as g/mol and the Na should also be in grams
Number of moles =
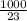 = 43.48mol
= 43.48mol
Number of moles of Br₂ =
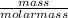
Number of moles =
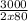 = 18.75mol
= 18.75mol
From the balanced reaction equation:
2 mole of Na combines with 1 mole of Br₂
43.48mole of Na would require 43.48/2 mole = 21.74mole of Br₂ to react with.
But the given amount of Br we have is 18.75mole.
Therefore, the reagent in short supply is Bromine. It is the limiting reagent
Quantity of NaBr formed
To find the quantity of NaBr formed, we use the known reagent which is the limiting one to determine its number of moles and eventually the mass. The limiting reagent determines the extent of the reaction.
From the stoichiometric equation:
1 mole of Br₂ will produce 2 mole of NaBr
19.23 mole Br₂ would now yield (18.75 x2)= 37.5mole of NaBr
Mass of NaBr = number of moles of NaBr x molar mass of NaBr
Molar mass of NaBr = 23 + 80 = 103g/mol
Mass of NaBr = 37.5 x 103 = 3862.5g = 3.86Kg
Amount of excess reagent remaining after the complete reaction:
The amount of excess reagent would be derived from the reagent that occurs in excess which is Na:
1 mole of Br₂ reacts with 2 moles of Na
18.75mole of Br₂ will require 2x 18.75 = 37.5mole of Na
Excess mole of Na = (43.48 - 37.5)mole = 5.98mole
Mass of excess Na = number of moles of excess Na x molar mass of Na
Mass of excess Na = 5.98 x 23
Mass of excess Na = 137.54g or 0.14g