Mg(s)+2HCl(aq)→MgCl2(aq)+H2(g)
(since the molar mass of Mg is not given, assuming that it's mola mass is 24gmol¯1)
1st find the moles of Mg using the equation n=m/M where
n - moles
m - mass
M - molar mass
Therefore :
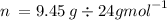
n = 0.39375mol
n = 0.39mol
Then using the equation n=V/Vm where;
n = mol
V = volume
Vm = molar volume
Find the volume.
n = V/Vm
(n =0.39mol , Vm = 22.4dm³mol¯¹)
V = 0.39mol×22.4dm³mol¯¹
V = 8.736dm³