Answer: The moles of nitrogen and hydrogen gas formed are 0.261 moles and 0.783 moles respectively.
Step-by-step explanation:
We are given:
Moles of ammonia = 0.261 moles
The chemical equation for the decomposition of ammonia follows:
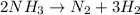
By Stoichiometry of the reaction:
2 moles of ammonia produces 1 mole of nitrogen gas and 3 moles of hydrogen gas
So, 0.261 moles of ammonia will produce
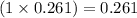 moles of nitrogen gas and
moles of nitrogen gas and
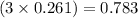 moles of hydrogen gas
moles of hydrogen gas
Hence, the moles of nitrogen and hydrogen gas formed are 0.261 moles and 0.783 moles respectively.