Answer:
The equations are
1)
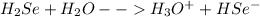
2)

Step-by-step explanation:
There are two ionization steps in the dissociation of hydroselenic acid.
In first dissociation the H₂Se loses one proton and forms hydrogen selenide ion as shown below:
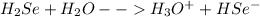
The next step is again removal of a proton from the base formed above.
