Answer:
The new volume of the gas is 1955.65 L.
Step-by-step explanation:
Initial temperature of the gas in the balloon =
 = 22°C = 295.15 K
= 22°C = 295.15 K
Initial volume of the the gas
 = 1820 L
= 1820 L
Final temperature of the gas in the balloon
 = 84°C = 317.15 K
= 84°C = 317.15 K
Final volume of the the gas =
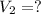
Under constant pressure and amount of gas, the gas will follow Charles' law:
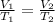 (Constant pressure)
(Constant pressure)
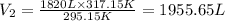
The new volume of the gas is 1955.65 L.