Answer:
Partial pressure of nitrogen gas,
![p^o_{N_2] =0.44 atm](https://img.qammunity.org/2020/formulas/physics/college/uglxz9np7v2b88ljzv3j8bez0xaz7llif3.png)
Step-by-step explanation:
Pressure of the mixture of gases before adding nitrogen gas = 0.85 atm
Pressure of the mixture of gases after adding nitrogen gas = 988 mmHg
1 mmHg = 0.001315 atm
988 mmHg=
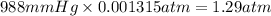
Partial pressure of nitrogen gas,
![p^o_{N_2] = 1.29 atm - 0.85 atm = 0.44 atm](https://img.qammunity.org/2020/formulas/physics/college/b5wou5flc80vnfvg7kc9htwy1ogjxqu1gu.png)
According to Dalton's law of partial pressure , the total pressure of the mixture of gases is equal to sum of all the partial pressures of each gas present in the mixture.
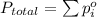
So, on addition of nitrogen gas to the mixture the pressure of the mixture increases.