Answer:
The increases temperature is 28.66°C.
Step-by-step explanation:
Given that,
Efficiency = 25.0%
Intensity = 1000 W/m^2
1 h = 3600 sec
Area
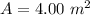
We need to calculate the power
Using formula of intensity
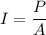
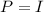
Where, P = power
I = intensity
A = area
Put the value into the formula
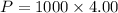
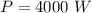
We need to calculate the temperature
Now, Using formula of power
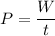
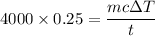
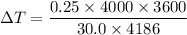
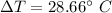
Hence, The increases temperature is 28.66°C.