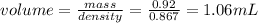Answer:
1.06 mL of toluene will be needed.
Step-by-step explanation:
Equi-molar mixture means equal moles of all the components.
as given the volume of ethyl acetate = 1mL
Density of ethyl acetate = 0.898 g/mL
The relation between density, mass and volume is :
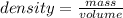
mass=volumeXdensity
mass of ethyl acetate present = 1mL X 0.898g/mL = 0.598 grams
the moles are related to mass as:
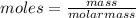
For ethyl acetate molar mass = 4X12+8X1+2X16= 88g/mol
moles of ethyl acetate will be:
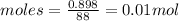
So we need 0.01 moles of toluene also
For 0.01 moles the mass of toluene required = 0.01 X molar mass of toluene
mass required = 0.01 X 92=0.92grams
for 0.92 grams of toluene volume required will be: