Answer:
245°C
Step-by-step explanation:
Properties of steam at 90°C
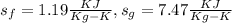
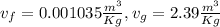
to find dryness fraction
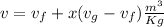
v is the specific volume,
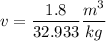
0.0546=0.001035+x(2.39-0.001035)
x=0.0224
When all water will covert in vapor steam then
volume
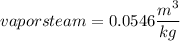
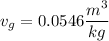
Now from steal table we can say that temperature corresponding to vapor volume
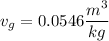 is 245°C.
is 245°C.