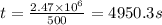Answer:
Part a)
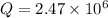
Part b)
t = 4950.3 s
Step-by-step explanation:
As we know that heat required to raise the temperature of container and water in it is given as
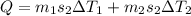
here we know that
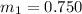
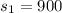
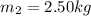
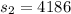
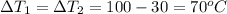
now we have
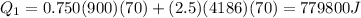
now heat require to boil the water
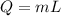
here
m = 0.750 kg
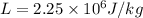
now we have
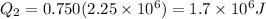
Now total heat required is given as
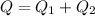
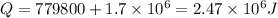
Part b)
Time taken to heat the water is given as
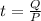
here we know that
power = 500 W
now we have