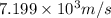Answer: The speed of hydrogen molecule is
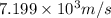
Step-by-step explanation:
The equation used to calculate the root mean square speed of a molecule is:
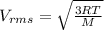
where,
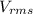 = root mean square speed of the molecule = ?
= root mean square speed of the molecule = ?
R = Gas constant = 8.314 J/mol.K
T = temperature = 4200 K
M = molar mass of hydrogen molecule =
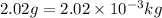 (Conversion factor: 1 kg = 1000 g)
(Conversion factor: 1 kg = 1000 g)
Putting values in above equation, we get:

Hence, the speed of hydrogen molecule is