Answer:
The molar mass of the unknown compound is 202.40 g/mol.
Step-by-step explanation:
Let the molar mass of compound be M.
The depression in freezing point is given by:
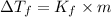
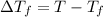
where,
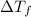 = change in boiling point = 0.81 K
= change in boiling point = 0.81 K
 =Boiling point of the solution = 3.77°C
=Boiling point of the solution = 3.77°C
T = freezing point of the pure solvent here benzene =5.48°C
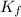 = freezing point constant = 5.12°C/m
= freezing point constant = 5.12°C/m
m = molality =
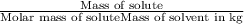
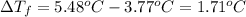

M = 202.40 g/mol
The molar mass of the unknown compound is 202.40 g/mol.