Answer : The time taken for the concentration will be, 7.98 seconds
Explanation :
First order reaction : A reaction is said to be of first order if the rate is depend on the concentration of the reactants, that means the rate depends linearly on one reactant concentration.
Expression for rate law for first order kinetics is given by :
![k=(2.303)/(t)\log([A]_o)/([A])](https://img.qammunity.org/2020/formulas/physics/college/1cw8rhcz4pvib041h138gcrlbe5p6vbef3.png)
where,
k = rate constant =
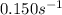
t = time taken for the process = ?
![[A]_o](https://img.qammunity.org/2020/formulas/chemistry/college/vfm07rqugmdqisio26k7clnl28e24vlfox.png) = initial concentration = 0.860 M
= initial concentration = 0.860 M
![[A]](https://img.qammunity.org/2020/formulas/chemistry/college/ey5pxctwpmy356s81f6qc2pjl6oveugako.png) = concentration after time 't' = 0.260 M
= concentration after time 't' = 0.260 M
Now put all the given values in above equation, we get:
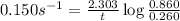
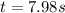
Therefore, the time taken for the concentration will be, 7.98 seconds