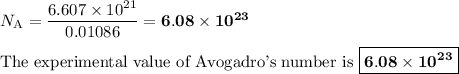Answer:
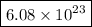
Step-by-step explanation:
Data:
I = 2.15 A
t = 8 min 24 s
T = 26.0 °C
V = 65.4 mL
p = 774.2 To
1. Write the equation for the half-reaction
2H₂O ⟶ O₂ + 4H⁺ + 4e⁻
2. Calculate the moles of oxygen
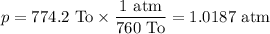
V = 0.0654 L
T = (26.0 + 273.15) K = 299.15 K
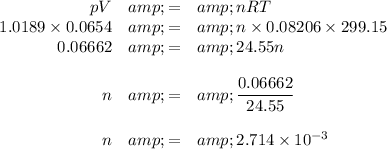
3. Calculate the moles of electrons
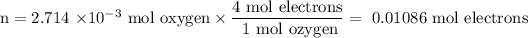
4. Calculate the number of coulombs
t = 8 min 24 s =504 s
Q = It = 504 s × 2.10 C·s⁻¹= 1058 C
5. Calculate the number of electrons
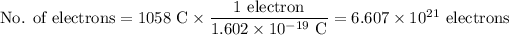
6. Calculate Avogadro's number