Answer:
The coefficient for the hydrochloric acid is 6.
Step-by-step explanation:
Stoichiometric coefficient are the number written in front of the reactants and products in balanced chemical reaction.
When solid aluminum reacts with hydrochloric acid to give aluminum chloride and hydrogen gas.
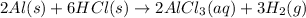
The coefficient for the aluminum is 2.
The coefficient for the hydrochloric acid is 6.
The coefficient for the aluminum chloride acid is 2.
The coefficient for the hydrogen gas is 3.